What Explains Why Molecules Have Different Dipolemoments
Dipole - induced dipole interactions are common in nonpolar substances. A neutral atom and an ion of the same element.

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps
This explains how two noble gases molecules can have an attractive force between them.

. For instance the water molecule shown in figure 1 has an electric dipole moment of 185 D and HCl has 109 D. Example PageIndex1 Dipole-Dipole Forces and Their Effects Predict which will have the higher boiling point. The volume of a container of hydrogen sulfide is 442mL.
So the molecules mentioned are either selenium. Another clue that a molecule is polar is the presence of lone pairs. For molecules there are three types of dipoles.
If the net dipole moment is zero or very very small the bond and molecule are considered to be nonpolar. Explain why H 2O is a polar molecule but H 2S is not. So Cillian try oxide.
N 2 or CO. The larger the difference in electronegativity between the two atoms the more electronegative that bond is. Lillian Dioxide which looked like this.
This is because for London Dispersion Forces to happen an atom just must have an electron but to have a permanent dipole certain conditions must be fulfilled like a high difference in electronegitivity. In chemistry dipole moments are applied to the distribution of electrons between two bonded atoms. Problem when 23 asks which of the molecules and problem 1 19 of a have net die pole moments or polish for modules is polar.
As shown in diagram. Such is the case with polar compounds like hydrogen fluoride HF where electron density is shared unequally between atoms. Molecules with a net dipole moment are polar molecules.
These occur when two atoms in a. To be considered a polar bond the difference in electronegativity must be large. Additionally we cannot attribute this difference in boiling points to differences in the dipole moments of the molecules.
Dispersion forces are known to arise as a result of temporary dipoles which arise in molecules and cause dipoles to become induced in other neutral molecules. Water is a good solvent due to its polarityWhen an ionic or polar compound enters water it is surrounded by water molecules. The dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together.
While molecules with zero dipole moment will have an asymmetrical shape. 43 Hydrogen sulfide gas H2S is a highly toxic gas that is responsible for the smell of rotten eggs. This explains why long hydrocarbon chains have relatively high boiling points.
Both molecules have about the same shape and ONF is the heavier and larger molecule. CO and N 2 are both diatomic molecules with masses of about 28 amu so they experience similar London dispersion forces. Many molecules have such dipole moments due to non-uniform distributions of positive and negative charges on the various atoms.
It is therefore expected to experience more significant dispersion forces. After the addition of more hydrogen sulfide the volume increases to. Arrow_forward Draw a molecule that has a trigonal pyramidal geometry using D as the central atom and G as the outer atoms picture does not have to show 3 dimensions and outer atoms do not need ione pairs.
The debye is of such magnitude that most molecules have dipole moments on the order of 1 to 10 D. Molecules with zero dipole moment are non-polar while molecules with dipole moment are said to be polar. Molecules that have an uneven distribution of charge and a dipole moment are called polar molecules.
A good example is the dipole moment of the water molecule. Pump is turned on. In finding the structure shape of the molecules.
In both cases the direction of the dipole is determined by the fact that the hydrogen atoms isare slightly positive. The existence of a dipole moment is the difference between polar and nonpolar bonds. Due to greater Electronegativity difference between atoms in a molecule the covalent bond between these two atoms becomes polar that means two oppositely charged poles are appeared.
Finally all molecules have London Dispersion Forces between them but not all will have a permanent dipole. Why water is a good solvent for ionic substances. After several minutes the volume of the water has decreased and what remains has turned into ice.
Why dont all molecules with polar covalent bonds have dipole moments. A positive pole of one molecule is attracted by the near by negative poles of another molecule. This explains how two noble gases molecules can have an attractive force between them.
Molecules with specific dipole moment values will be bent or angular in shape and not have a symmetrical structures. Even though the total charge on a molecule is zero the nature of chemical bonds is such that the positive and negative charges do not completely overlap in most molecules. This explains how the molecules hydrogen fluoride and methanol can exhibit uncharacteristically strong intermolecular forces.
Describe the similarities and differences between. Temporary dipole moments explains how two noble gases molecules can have an attractive force between them. Because CO is a polar molecule it experiences dipole-dipole attractions.
Here is a tribunal plainer molecule um with essentially the central selenium and three surrounding oxygens. This explains why long. Different isotopes of an element b.
Such molecules are said to be polar because they possess a permanent dipole moment. This explains why the dipole dipole attractive force between dimethyl ether and acetone does not entirely account for the attractive force between these molecules. Both molecules are polar and exhibit comparable dipole moments.

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube
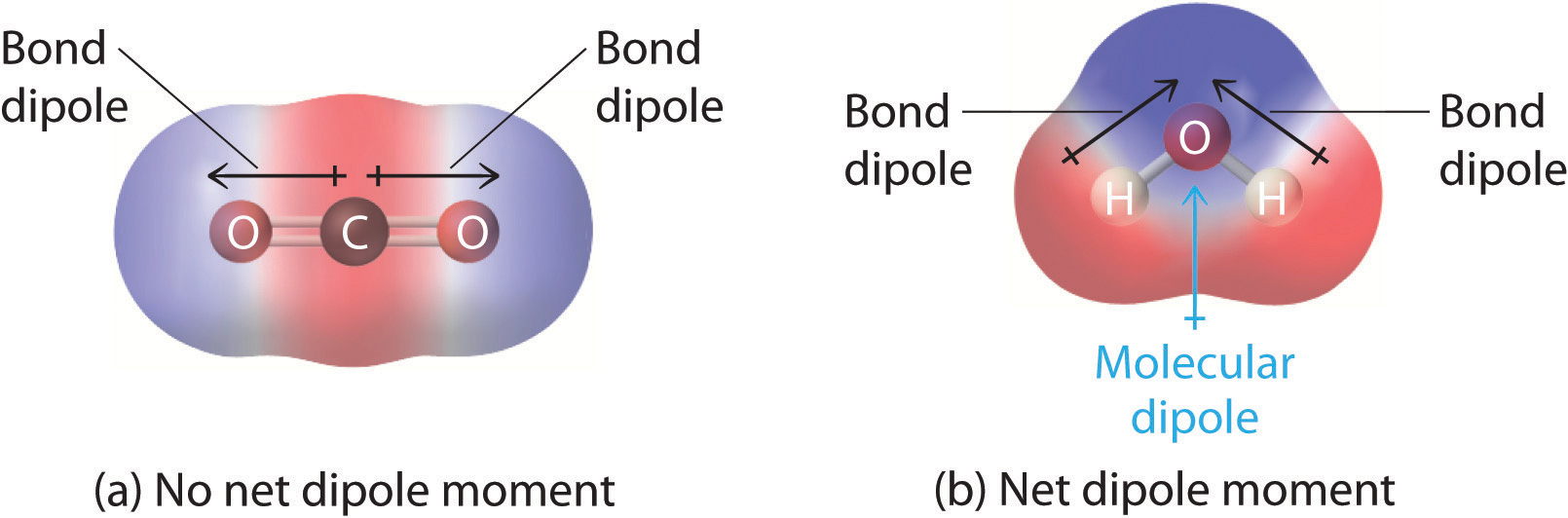
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps
No comments for "What Explains Why Molecules Have Different Dipolemoments"
Post a Comment